Pennsylvania's biotechnology and pharmaceutical sectors require specialized background screening protocols. These extend beyond standard employment verification. They must address FDA compliance, controlled substance regulations, and intellectual property protection mandates. Companies operating in Pennsylvania's biotech corridors navigate a complex regulatory framework. This includes federal FCRA requirements, state-specific licensing verification, and industry-specific security standards.
Key Takeaways
- Pennsylvania biotech background check requirements encompass federal FDA regulations, state licensing verification, and industry-specific security protocols for companies handling controlled substances and sensitive research data.
- Standard screening components include criminal history checks at state and federal levels, education verification, professional licensing validation, and reference checks tailored to laboratory environments.
- FDA-regulated facilities must implement enhanced screening procedures for employees with access to drug manufacturing operations, clinical trial data, and proprietary pharmaceutical formulations.
- Pennsylvania state law requires specific background checks for positions involving controlled substances, with additional scrutiny for Schedule II-V drug handlers and pharmacy technicians.
- Companies in Philadelphia, Pittsburgh, and State College biotech hubs face heightened compliance expectations due to concentrated research institutions and pharmaceutical manufacturing facilities.
- FCRA compliance mandates proper disclosure, authorization, and adverse action procedures, with specific considerations for continuous monitoring programs in high-security biotech environments.
- Background screening timelines typically range from 3-10 business days for standard checks, with extended periods for international education verification and federal security clearances.
- Implementing comprehensive background screening programs reduces regulatory violations, protects intellectual property, and maintains operational integrity in Pennsylvania's competitive life sciences marketplace.
Understanding Pennsylvania's Biotech Industry Regulatory Landscape
Pennsylvania ranks among the top five states for pharmaceutical manufacturing and biotechnology research. The state hosts over 650 life sciences companies employing approximately 90,000 professionals as of 2025. The Commonwealth's strategic position in the Mid-Atlantic corridor creates a robust ecosystem. World-class research institutions like the University of Pennsylvania and Carnegie Mellon University strengthen this foundation. Pennsylvania biotech background check requirements reflect this unique regulatory environment. Federal oversight, state mandates, and industry best practices converge here.
The state's biotech sector encompasses diverse operations. These range from early-stage drug discovery to large-scale pharmaceutical manufacturing. Each operation type has distinct screening requirements. Companies conducting clinical research verify credentials differently than those in biologics production. This regulatory complexity demands specialized knowledge. Pennsylvania pharmaceutical background screening standards address both employment law and industry-specific compliance obligations.
Pennsylvania biotech employers operate under multiple jurisdictions simultaneously. Federal agencies like the FDA and DEA establish baseline standards for controlled environments. Pennsylvania state law adds specific provisions for healthcare-adjacent positions and controlled substance handlers. Local municipalities may impose additional requirements. This is particularly true in Philadelphia and Pittsburgh. Biotech companies concentrate near major medical centers and research universities in these cities.
Federal Compliance Requirements for Pennsylvania Biotech Companies
FDA Background Check Compliance Standards
The Food and Drug Administration establishes comprehensive personnel screening requirements. These apply to facilities engaged in drug manufacturing, clinical trials, and biologics production. Pennsylvania FDA background check compliance obligations apply to companies operating under current Good Manufacturing Practice (cGMP) regulations. These regulations mandate documented procedures for evaluating employee qualifications and trustworthiness. The requirements specifically target positions with access to manufacturing operations, quality control laboratories, and areas where product integrity could be compromised.
FDA guidance documents emphasize verifying education, training, and experience. This applies to personnel performing critical manufacturing and quality assurance functions. Companies must maintain records demonstrating that employees possess appropriate qualifications. This must occur before assignment to regulated activities. For Pennsylvania biotech companies, this translates to enhanced verification procedures. These apply to formulation scientists, quality control analysts, validation engineers, and production supervisors. All work in FDA-registered facilities.
The FDA's focus on data integrity has intensified scrutiny. This applies to personnel with access to laboratory information management systems and electronic batch records. Pennsylvania pharmaceutical companies must implement screening procedures. These assess candidates' history of regulatory compliance, academic integrity, and professional conduct. This extends beyond criminal background checks. It includes verification of scientific credentials, review of publication records, and validation of previous employment in regulated environments.
DEA Controlled Substance Regulations
The Drug Enforcement Administration imposes strict requirements on facilities handling controlled substances. These requirements span from research-grade compounds to commercial pharmaceutical products. Pennsylvania biotechnology companies working with Schedule II through V substances must conduct thorough background investigations. These apply to employees with access to secure storage areas, manufacturing suites, and distribution systems. These pharmaceutical research background verification procedures help prevent diversion, theft, and unauthorized access.
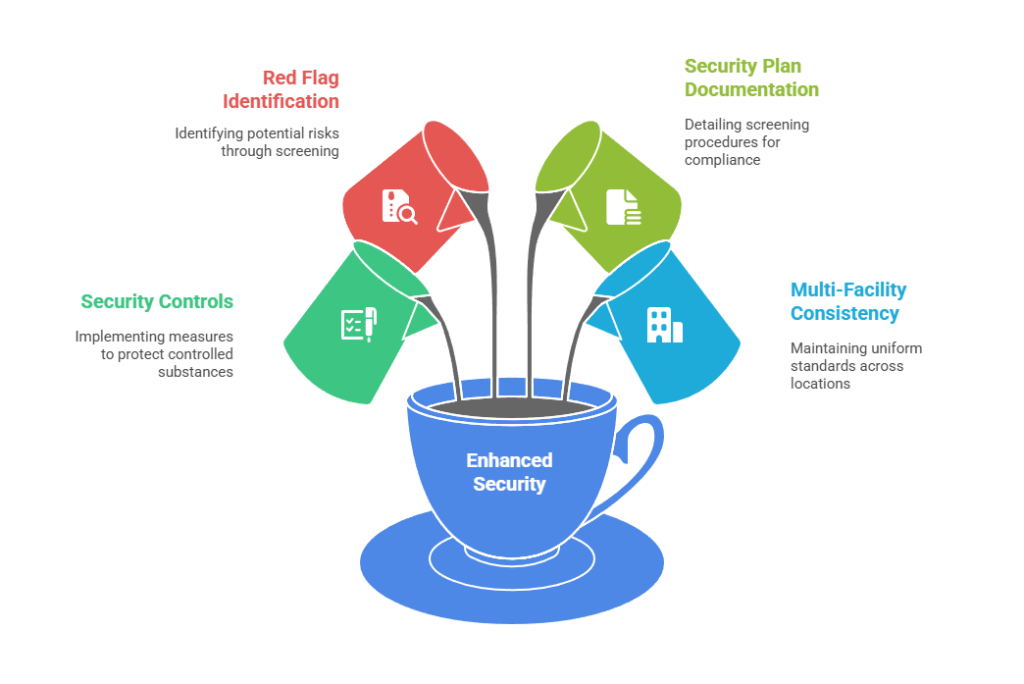
- Security controls implementation: DEA regulations require registrants to implement security controls commensurate with the schedule and quantity of controlled substances on-site. Pennsylvania facilities conducting opioid research or manufacturing pain management medications need comprehensive employment screening. This examines criminal history related to drug offenses, financial irregularities, and previous employment terminations.
- Red flag identification: The screening process should identify red flags. These include undisclosed criminal convictions, falsified credentials, or patterns of behavior suggesting substance abuse issues that could compromise controlled substance security.
- Security plan documentation: Pennsylvania biotech companies must document their screening procedures in security plans. These are submitted to DEA during registration and renewal processes. Plans detail the specific background check components, the positions subject to enhanced screening, and the decision-making criteria for determining employee suitability.
- Multi-facility consistency: Companies operating multiple facilities across Pennsylvania must ensure consistent application of screening standards. They must also account for site-specific risk factors based on the controlled substances present at each location.
These comprehensive DEA-mandated screening procedures form a critical component of Pennsylvania pharmaceutical background screening programs. They protect both regulatory compliance and public safety.
Pennsylvania State-Specific Background Screening Requirements
Pennsylvania law establishes specific background check requirements. These apply to healthcare and pharmaceutical positions that intersect with biotech industry operations. The state's screening mandates apply primarily to direct patient care roles. However, many provisions extend to laboratory personnel, pharmacy technicians, and other positions. Pittsburgh biotech company screening requirements often reflect these healthcare standards. This occurs due to integration of research facilities with major medical centers like UPMC and Allegheny Health Network.
The Pennsylvania State Police maintain criminal history repositories. Employers must access these for positions involving vulnerable populations or controlled substances. Companies can request state-only criminal background checks. They can also combine Pennsylvania records with FBI fingerprint-based searches for comprehensive national coverage. The state requires specific consent forms. It imposes restrictions on how criminal history information can be used in employment decisions.
Pennsylvania's Clean Slate Law automatically seals certain criminal records. This impacts how biotech employers access and interpret background check results. Records of arrests not resulting in conviction become inaccessible through standard background checks. So do summary offenses more than five years old and certain misdemeanors. Biotech companies must design screening programs that account for sealed records. They must also maintain compliance with FDA and DEA requirements. This creates a challenging balance between state privacy protections and federal transparency mandates.
Professional Licensing Verification Standards
Pennsylvania biotechnology security clearance processes frequently require validation of professional licenses. These are issued by state regulatory boards. The State Board of Pharmacy oversees pharmacist and pharmacy technician credentials. These are essential for biotech companies with on-site dispensing operations or clinical trial drug distribution. Positions requiring Pennsylvania medical licenses, nursing credentials, or clinical laboratory certifications demand thorough verification. This occurs through state licensing databases.
The verification process extends beyond confirming active license status. It examines disciplinary history, practice restrictions, and continuing education compliance. Pennsylvania's licensing boards maintain public databases accessible for employment screening. However, comprehensive verification should include direct confirmation with the issuing authority. For clinical research background check in Pennsylvania, this means validating credentials. These apply to principal investigators, study coordinators, and laboratory directors who hold professional licenses critical to research integrity.
Healthcare Worker Background Check Mandates
Pennsylvania law requires criminal background checks for healthcare workers. This applies to those who have direct contact with patients or access to patient records. This requirement affects biotech companies conducting clinical trials or operating patient-facing research facilities. The requirements apply to contract research organizations managing trial sites. They also apply to pharmaceutical companies sponsoring studies with direct patient interaction. Biotech firms with integrated clinical operations must comply as well. The screening must include Pennsylvania State Police criminal history checks. It must also include FBI fingerprint-based searches for comprehensive national coverage.
The healthcare worker background check process examines specific offense categories. These create automatic disqualifications or trigger individualized assessments. Convictions for crimes against vulnerable populations typically prevent employment in covered positions. So do drug trafficking, healthcare fraud, and violent felonies. Pennsylvania law requires employers to evaluate the nature of the offense. They must consider time elapsed and evidence of rehabilitation. This evaluation occurs before making adverse employment decisions based on criminal history.
Essential Components of Pennsylvania Biotech Background Checks

A comprehensive background screening program for Pennsylvania pharmaceutical companies incorporates multiple verification elements. These are tailored to industry-specific risks. The standard framework includes criminal history searches at county, state, and federal levels. Companies pay particular attention to offenses involving fraud, theft, violence, and drug-related crimes. Given the intellectual property value and regulatory scrutiny in biotech environments, Pennsylvania employers typically conduct more extensive screening. This exceeds minimum legal standards.
Education verification forms a critical component for positions requiring scientific expertise. Companies emphasize confirming degrees from institutions claimed on applications and resumes. Pennsylvania's concentration of prestigious universities produces numerous biotech candidates. However, credential fraud remains a persistent concern. Verification should extend beyond degree confirmation. It should include dates of attendance, majors, and graduation status. Particular scrutiny applies to advanced degrees in molecular biology, biochemistry, pharmaceutical sciences, and related disciplines.
Employment history verification provides insights into candidates' professional trajectory, stability, and performance. Pennsylvania biotech employers should focus verification efforts on the most recent 7-10 years of employment. They emphasize positions involving similar responsibilities, regulatory environments, or access to sensitive information. Reference checks supplement documented employment verification. They explore candidates' technical competencies, regulatory compliance awareness, and suitability for positions requiring attention to detail and ethical conduct.
Criminal History Screening Parameters
Pennsylvania biotechnology companies should conduct multi-jurisdictional criminal background checks. These capture offense records across relevant geographic areas. A state-only Pennsylvania criminal history check provides conviction records reported to the Pennsylvania State Police. However, it may miss offenses from other states where candidates lived or worked. National database searches cast a wider net but rely on reporting consistency. They may contain outdated or incomplete information requiring verification through county court records.
| Screening Level | Coverage Area | Typical Timeline | Best Used For |
| Pennsylvania State Check | PA convictions only | 1-3 business days | Minimum compliance, Pennsylvania-only work history |
| FBI Fingerprint Check | National convictions | 3-5 business days | Federal compliance positions, security clearances |
| National Database Search | Multi-state records | 1-2 business days | Initial screening, identifying search jurisdictions |
| County Court Records | Specific county convictions | 2-5 business days per county | Comprehensive verification of work history addresses |
The appropriate criminal screening strategy depends on position sensitivity, regulatory requirements, and candidates' geographic history. Pennsylvania pharmaceutical background screening for executive roles and principal investigators should include FBI fingerprint checks. Positions with controlled substance access require targeted county searches. Entry-level positions with limited access to sensitive areas might appropriately rely on Pennsylvania state checks. These can be supplemented by national database searches.
Professional Reference and Performance Verification
Reference checks for Pennsylvania biotech positions should target supervisors and colleagues with direct knowledge. They assess candidates' technical skills, regulatory compliance awareness, and ethical conduct. Standard reference questions about attendance and eligibility for rehire provide limited value. Targeted inquiries about laboratory practices offer more insight. So do questions about attention to documentation requirements and response to quality issues. Pennsylvania pharmaceutical companies benefit from developing position-specific reference questionnaires. These explore competencies critical to research integrity and manufacturing excellence.
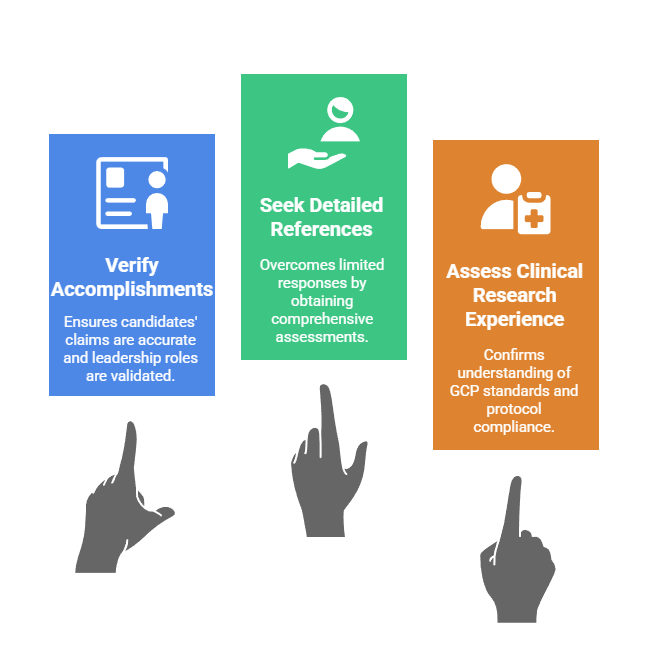
- Accomplishment validation: The reference verification process should confirm candidates' claimed accomplishments, project roles, and technical responsibilities. It should not accept self-reported descriptions. Senior scientific positions require validation of leadership of specific research programs. This includes authorship of publications and contributions to regulatory submissions.
- Overcoming limited responses: Many companies limit reference information to dates of employment and title due to litigation concerns. This makes it essential to request references from individuals candidates identify as willing to provide detailed assessments. These should cover work performance and capabilities.
- Clinical research focus: Pennsylvania biotech employers conducting clinical research should specifically explore candidates' understanding of Good Clinical Practice standards. They should assess experience with institutional review board processes and history of protocol compliance. This occurs through discussions with references from previous trial sites or contract research organizations.
This specialized reference verification complements standard background checks. It assesses competencies that criminal history and education verification cannot capture. It provides valuable insights into how candidates will perform in highly regulated pharmaceutical environments.
Advanced Screening for High-Security Biotech Positions
Pennsylvania biotechnology facilities handling high-value intellectual property require enhanced screening protocols. So do those working with advanced biologics or sensitive government contracts. These protocols extend beyond standard employment verification. Pittsburgh biotech company screening requirements often include security clearance investigations, financial background reviews, and social media assessments. These measures are designed to identify potential insider threats. Companies engaged in Department of Defense biodefense research face additional federal screening mandates. So do those handling controlled select agents.
Credit history checks provide insights into financial pressures. These might motivate intellectual property theft or data compromise. However, Pennsylvania employers must carefully limit their use. They should apply only to positions with financial responsibilities or access to valuable proprietary information. The screening should focus on patterns of financial instability, major judgments, or bankruptcies. It should not emphasize minor credit issues. Companies must provide proper FCRA disclosures before obtaining credit reports. For positions involving trade secret access, financial screening helps assess susceptibility to competitive intelligence approaches.
Social media and online presence reviews offer supplementary information. They reveal candidates' judgment, professional reputation, and potential conflicts of interest. Pennsylvania pharmaceutical companies should establish clear policies governing social media screening. These policies ensure consistent, non-discriminatory application and compliance with privacy expectations. The review should focus on publicly available information relevant to job suitability. Companies should avoid protected class information and personal matters unrelated to employment.
Intellectual Property and Confidentiality Assessments
Pennsylvania biotech companies protecting valuable drug candidates should screen for indicators of intellectual property risk. So should those protecting manufacturing processes or research methodologies. This screening includes reviewing candidates' patent filings, publication histories, and previous employment at competing organizations. The goal is to identify potential confidentiality conflicts. Pennsylvania pharmaceutical background screening for positions with trade secret access should examine obligations to former employers. These obligations might restrict candidates' ability to work on specific projects or technology platforms.
Non-compete agreements and confidentiality obligations from previous employment create particular challenges. This is especially true in Pennsylvania's competitive biotech landscape. While Pennsylvania courts generally enforce reasonable restrictive covenants, the analysis requires fact-specific assessment. This considers geographic scope, duration, and competitive overlap. Background screening should identify existing agreements requiring legal review before extending offers. This protects the hiring company from discovery litigation. It also protects candidates from being placed in compromising positions.
International Background Screening Considerations
Pennsylvania's biotech workforce includes significant numbers of international scientists, researchers, and technical specialists. These professionals require background verification in home countries and previous work locations. International screening presents unique challenges. These include varying record availability, different legal frameworks governing information access, and extended timelines. Companies should partner with background screening providers experienced in international verification. These providers understand country-specific requirements. They can navigate language barriers, data protection regulations, and authentication procedures.
Education verification for international degrees requires specialized processes. These confirm institutional legitimacy and credential equivalency to U.S. standards. Pennsylvania pharmaceutical companies hiring scientists trained abroad should verify degrees through credential evaluation services. These services assess educational qualifications against American benchmarks. This proves particularly important for positions requiring specific scientific knowledge. It also applies to regulatory submissions listing personnel qualifications. Degree equivalency must be documented for FDA inspections.
FCRA Compliance in Pennsylvania Biotech Hiring
The Fair Credit Reporting Act establishes comprehensive requirements for employers using consumer reports. This includes background checks in employment decisions. Pennsylvania biotech companies must provide clear written disclosure that background checks will be conducted. They must also obtain standalone written authorization before requesting reports from consumer reporting agencies. The disclosure and authorization must be separate documents. They cannot be buried in employment applications or acknowledgment forms. This separation ensures candidates understand they are authorizing background screening.
FCRA compliance requires specific procedures when employers intend to take adverse action. Pennsylvania pharmaceutical companies must provide pre-adverse action notice. This includes a copy of the consumer report and a summary of FCRA rights before making final decisions. This allows candidates to review findings, identify inaccuracies, and dispute incorrect information. They can do this before losing employment opportunities. After providing reasonable time for dispute, typically 5-7 business days, employers can proceed with adverse action. They can do this if they confirm the information is accurate and relevant.
Penalties for FCRA violations include statutory damages, actual damages, punitive damages, and attorney fees. These penalties create significant financial exposure for non-compliant companies. Pennsylvania biotechnology employers should implement documented procedures ensuring consistent FCRA compliance. This applies across all background checks regardless of position level or screening complexity. Procedures include training hiring managers on disclosure requirements. They also include establishing adverse action processes and maintaining records.
Disclosure and Authorization Requirements
Pennsylvania biotech companies must provide clear, conspicuous disclosure of background screening practices. This must occur before conducting checks through consumer reporting agencies. The disclosure should identify the types of reports that may be obtained. These include criminal history, employment verification, education confirmation, and credit reports if applicable. The document must be standalone. It cannot be combined with employment applications, offer letters, or other hiring materials. However, it can be in electronic format if the candidate applies online.
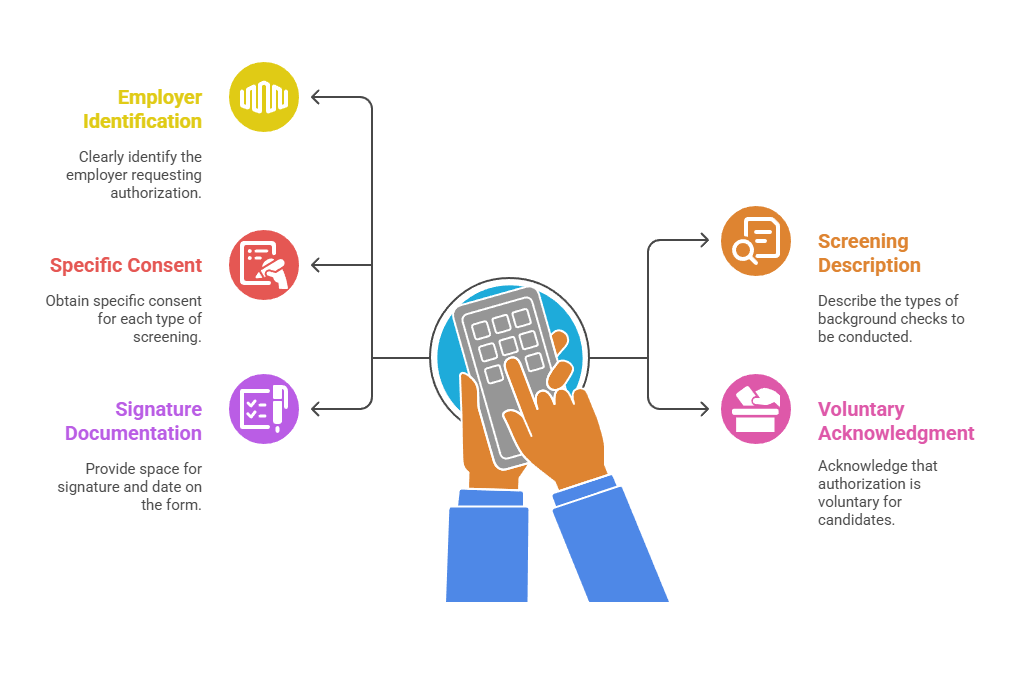
- Employer identification: Authorization forms must clearly identify the employer requesting authorization. This establishes the formal relationship between the company and the candidate being screened.
- Screening type description: The form should describe the types of background checks that will be conducted. This provides transparency about what information will be gathered and reviewed.
- Specific consent requirements: Companies must obtain specific consent for each type of screening rather than blanket authorization. This ensures candidates understand exactly what they're authorizing.
- Voluntary acknowledgment: The authorization should include acknowledgment that authorization is voluntary. However, candidates understand it may be required for employment consideration.
- Signature documentation: Forms must provide space for signature and date. This creates a legally binding record of candidate consent that satisfies FCRA requirements.
Companies should maintain signed disclosure and authorization forms for the duration of employment plus at least five years. This demonstrates FCRA compliance if disputes arise. Pennsylvania biotech employers using applicant tracking systems should configure workflows carefully. These should prevent progression through hiring stages until proper disclosures and authorizations are obtained and documented.
Adverse Action Procedures
When background check results reveal information that may lead to withdrawal of employment offers or termination, Pennsylvania employers must follow specific adverse action procedures. The process begins with a pre-adverse action notice. This provides candidates copies of consumer reports, summaries of FCRA rights, and reasonable opportunity to dispute inaccurate information. This step is critical for Pennsylvania biotechnology companies. Background check errors occur with sufficient frequency. Premature adverse action based on incorrect information creates significant legal exposure.
The waiting period between pre-adverse action notice and final decision should provide meaningful opportunity. Candidates need time to identify errors and initiate disputes with consumer reporting agencies. While FCRA does not mandate specific timeframes, five to seven business days represents industry best practice. Courts generally view this period as reasonable. Pennsylvania pharmaceutical companies should document the date pre-adverse action materials were provided. They should maintain evidence of delivery. This demonstrates compliance if candidates later claim insufficient opportunity to respond.
Implementing Continuous Monitoring Programs
Pennsylvania biotechnology companies increasingly adopt continuous criminal monitoring. This identifies arrests, convictions, or professional license sanctions occurring after initial employment. These ongoing background check programs help maintain compliance with FDA personnel qualification requirements. They also help meet DEA security obligations for employees with controlled substance access. Continuous monitoring provides alerts when monitored employees encounter law enforcement. This enables timely response to situations that might affect job suitability or regulatory compliance.
Implementing continuous monitoring requires additional FCRA disclosures and authorizations. These extend beyond those used for pre-employment screening. Pennsylvania pharmaceutical companies must clearly explain that background checks will continue throughout employment. They must describe the types of monitoring conducted and obtain specific consent for ongoing screening. The disclosure should address how monitoring information will be used. It should also cover retention periods and circumstances triggering individualized review of reported incidents.
Continuous monitoring generates alerts for various record updates. These include new criminal charges, convictions, sex offender registrations, and professional license changes. Pennsylvania biotech employers must establish protocols for reviewing alerts and determining appropriate responses. The response depends on offense severity, position requirements, and regulatory implications. Not every alert warrants employment action. Companies should implement individualized assessment procedures rather than automatic termination policies.
Balancing Security Needs with Privacy Considerations
Continuous monitoring programs must balance legitimate security interests with employee privacy expectations and legal constraints. Pennsylvania biotechnology employers should limit monitoring to positions where ongoing screening is justified. Justification comes from security requirements, regulatory mandates, or access to sensitive information. Monitoring all employees regardless of role creates unnecessary privacy intrusions. It also creates administrative burden without proportional security benefits.
The monitoring program should clearly define which positions are subject to continuous screening. It should also specify the exact checks conducted. For Pennsylvania FDA background check compliance, this typically includes positions in manufacturing operations. It also includes quality control laboratories and areas with drug product access. Research scientists, administrative personnel, and positions without controlled environment access might not require continuous monitoring. However, companies may voluntarily apply similar standards if these positions handle valuable intellectual property.
Best Practices for Pennsylvania Biotech Background Screening
Pennsylvania pharmaceutical companies should develop comprehensive background screening policies. These must address industry-specific risks and ensure consistent, legally compliant application across all candidates. The policy should clearly define screening requirements for different position categories. These range from entry-level laboratory technicians to senior executives. Companies should use enhanced protocols for roles involving controlled substances, intellectual property access, or regulatory responsibilities. Written policies provide frameworks for hiring managers. They also demonstrate systematic approaches if screening decisions are later challenged.
The screening policy should establish clear timelines for obtaining background checks. It should include checkpoints in the hiring process where specific verifications must be completed. Pennsylvania biotechnology security clearance processes require extended timeframes. This applies to comprehensive international screening and federal security investigations. Early initiation is necessary to avoid offer delays. Companies should communicate expected timelines to candidates. They should also maintain contingency plans for situations where background checks reveal issues requiring additional investigation.
Regular audits of background screening practices help Pennsylvania biotech employers identify compliance gaps. These audits ensure consistent policy application. The audit process should examine disclosure and authorization procedures. It should also review adverse action documentation, record retention practices, and alignment between written policies and actual implementation. Companies should conduct internal audits at least annually. They should consider external compliance reviews when screening programs expand or regulatory requirements change.
Vendor Selection and Quality Assurance
Pennsylvania pharmaceutical background screening programs depend on consumer reporting agencies and verification services. These vendors must themselves comply with FCRA and maintain quality standards. Companies should select vendors based on industry expertise, data source quality, turnaround times, and customer support capabilities. Vendors serving the biotech sector should understand FDA and DEA requirements. They should have experience with international verification. They should provide specialized services like professional license monitoring and scientific credential verification.
The vendor evaluation process should include reviewing FCRA compliance procedures, data security practices, and quality assurance methodologies. Pennsylvania biotech employers remain responsible for screening accuracy even when third-party vendors conduct verification. This makes vendor quality critical to overall compliance. Companies should request information about data sources and dispute resolution procedures. They should ask how vendors ensure information currency and completeness. These details help assess vendor reliability and suitability for biotech industry needs.
Vendor contracts should clearly define service levels, turnaround time expectations, and procedures for resolving accuracy disputes. The agreement should address data security requirements. This is particularly important for Pennsylvania pharmaceutical companies handling sensitive candidate information. Vendor performance should be monitored through metrics. These include on-time delivery rates, accuracy percentages, and candidate satisfaction scores. Regular business reviews ensure service quality remains acceptable. They also address any emerging issues promptly.
Conclusion
Pennsylvania biotech background check requirements reflect the high-stakes nature of pharmaceutical research, clinical trials, and drug manufacturing. Personnel integrity directly impacts product quality and regulatory compliance in these environments. Companies operating in Pennsylvania's life sciences corridors must implement comprehensive screening programs. These address federal FDA and DEA requirements, state-specific mandates, and industry best practices. The most effective programs balance thoroughness with efficiency. They ensure qualified candidates move through hiring processes expeditiously while maintaining appropriate verification standards. By investing in robust background screening infrastructure, Pennsylvania biotechnology and pharmaceutical companies protect their operations and maintain regulatory standing.
Frequently Asked Questions
What background checks are required for biotech employees in Pennsylvania?
Pennsylvania biotech companies must conduct criminal background checks, education verification, and professional license validation for positions involving FDA-regulated activities. This also applies to positions with controlled substance access or patient contact. Specific requirements vary based on position responsibilities. Enhanced screening applies to manufacturing operations and quality control roles. Companies should implement risk-based screening programs that exceed minimum legal requirements.
How long do Pennsylvania biotech background checks take to complete?
Standard background checks for Pennsylvania pharmaceutical positions typically require 3-7 business days. This includes state criminal history, education verification, and employment confirmation. More comprehensive screening with FBI fingerprint checks and international verification may extend to 2-3 weeks. Companies should initiate background checks early in hiring processes. They should communicate expected timelines, particularly for positions requiring federal security clearances.
Can Pennsylvania biotech companies conduct continuous criminal monitoring?
Yes, Pennsylvania employers can implement continuous monitoring programs with proper FCRA disclosures and candidate authorization. These programs provide ongoing alerts about criminal activity, professional license changes, and sex offender registrations for current employees. Continuous monitoring proves particularly valuable for positions with controlled substance access or FDA compliance requirements. Companies must establish individualized review procedures for monitoring alerts.
What criminal offenses disqualify candidates from Pennsylvania pharmaceutical jobs?
Pennsylvania law does not establish blanket disqualifications based on criminal history. However, FDA and DEA regulations create practical restrictions for certain convictions. Drug trafficking offenses, healthcare fraud, theft, and crimes involving deception typically disqualify candidates. This applies to positions with controlled substance access or FDA-regulated responsibilities. Employers must conduct individualized assessments considering offense severity, time elapsed, and rehabilitation evidence.
Are credit checks allowed for Pennsylvania biotech positions?
Pennsylvania employers can conduct credit checks for biotech positions with proper FCRA disclosure and authorization. However, they should limit use to roles with financial responsibilities or access to valuable intellectual property. Credit reports help assess financial pressures that might motivate proprietary information theft. Companies should focus on patterns of financial instability rather than minor credit issues.
Do Pennsylvania background check requirements differ for contract workers?
Pennsylvania pharmaceutical companies should apply similar background screening standards to contractors and temporary workers. This applies to those who will access controlled environments, handle sensitive research, or work in FDA-regulated areas. The company hosting contract workers maintains responsibility for ensuring personnel are qualified and trustworthy. Contracts with staffing agencies should specify background check requirements and verification documentation needed before contractors begin assignments.
What happens if a Pennsylvania biotech background check reveals errors?
FCRA requires employers to provide pre-adverse action notice with copies of background reports. This applies to information that may lead to employment denial. This allows candidates to dispute inaccuracies before final decisions. Candidates should contact the consumer reporting agency to initiate disputes. Pennsylvania biotech employers must wait reasonable periods, typically 5-7 business days, for dispute resolution before proceeding with adverse action.
How should Pennsylvania pharmaceutical companies handle background checks for international candidates?
Pennsylvania biotech employers hiring international scientists should conduct background screening in candidates' home countries and previous work locations. Record availability and verification processes vary significantly across jurisdictions. Companies should partner with background screening providers experienced in international verification. These providers can navigate country-specific requirements, language barriers, and authentication procedures. Education verification for foreign degrees should include credential evaluation to confirm equivalency to U.S. standards.
Additional Resources
- FDA Guidance for Industry: Personnel Qualifications
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guide-general-principles-process-validation - Pennsylvania Department of Health: Licensing and Certification
https://www.health.pa.gov/topics/programs/Pages/Licensing.aspx - DEA Diversion Control Division: Security Requirements
https://www.deadiversion.usdoj.gov/drugreg/index.html - Federal Trade Commission: Background Checks and FCRA Compliance
https://www.ftc.gov/business-guidance/resources/using-consumer-reports-what-employers-need-know - Pennsylvania State Police: Criminal Background Check Information
https://www.psp.pa.gov/Pages/default.aspx
Still have questions?
Get in touch with our team today for a personalized demo and discover how our tailored volume pricing and packages can drive results for your business!
How useful was this page?*
Note: your comments are anonymous. We use them to improve the website. Do not include any personal details.
Visit our FCRA Compliance Tool or leave a message here if you need a response.
From the blog Explore the GCheck Content Hub

How Long Does a Background Check Take? A Complete 2025 Guide
13 Dec, 2023 • 14 min read
The Ultimate Background Check Guide
13 Dec, 2023 • 4 min read
The Ultimate Guide to Employment Background Checks
13 Dec, 2023 • 10 min readThe information provided in this article is for general informational and educational purposes only and should not be construed as legal advice or a substitute for consultation with qualified legal counsel. While we strive to ensure accuracy, employment screening laws and regulations—including but not limited to the Fair Credit Reporting Act (FCRA), Equal Employment Opportunity Commission (EEOC) guidelines, state and local ban-the-box laws, industry-specific requirements, and other applicable federal, state, and local statutes—are subject to frequent changes, varying interpretations, and jurisdiction-specific applications that may affect their implementation in your organization. Employers and screening decision-makers are solely responsible for ensuring their background check policies, procedures, and practices comply with all applicable laws and regulations relevant to their specific industry, location, and circumstances. We strongly recommend consulting with qualified employment law attorneys and compliance professionals before making hiring, tenant screening, or other decisions based on background check information.

