Pharmaceutical industry hiring compliance requires far more rigorous employee screening than standard background checks, particularly for sales representatives who access controlled medication samples and interact directly with healthcare providers. With FDA enforcement actions increasing 23% in recent years and average regulatory penalties exceeding $2.8 million per violation, pharmaceutical companies must implement comprehensive screening protocols that address OIG exclusions, credential verification, fraud history, and ongoing monitoring to protect both patient safety and corporate reputation.
Key Takeaways
- Pharmaceutical sales representatives require specialized background checks beyond standard criminal history due to sample access privileges and direct healthcare provider interactions that create unique fraud and diversion risks.
- Monthly OIG exclusion list screening is mandatory for all pharmaceutical employees, with companies facing severe penalties including loss of federal healthcare program participation for employing excluded individuals.
- FDA compliance screening must verify professional licenses, educational credentials, and prior regulatory sanctions across multiple databases that standard employment verification processes typically miss.
- Pharmaceutical background checks should include seven-year criminal history searches, financial fraud investigations, motor vehicle records, and healthcare sanctions monitoring to address industry-specific risk factors.
- Companies that fail to implement adequate pharmaceutical employee screening requirements face average regulatory penalties of $2.8 million per violation, plus reputational damage and potential criminal prosecution.
- The pharma sales rep screening checklist must include ongoing monitoring components, as 34% of exclusions and sanctions occur after initial employment, requiring continuous compliance verification.
- Healthcare worker verification for pharmaceutical roles demands multi-jurisdictional searches including state medical boards, DEA registrations, and controlled substance monitoring programs beyond typical employment screening.
- Inadequate screening creates cascading liability where a single non-compliant hire can trigger company-wide compliance audits, increased regulatory scrutiny, and mandatory corporate integrity agreements lasting 3-5 years.
Why Pharmaceutical Industry Hiring Compliance Demands Specialized Screening
The pharmaceutical industry operates under one of the most stringent regulatory frameworks in American business. Unlike general corporate hiring, pharmaceutical industry hiring compliance intersects with patient safety, controlled substance access, and direct healthcare system interaction. This unique position creates compliance requirements that standard background screening cannot adequately address.
Pharmaceutical companies face regulatory oversight from multiple federal agencies simultaneously. The FDA, DEA, OIG, and Department of Justice each maintain distinct compliance mandates. A single hiring misstep can trigger investigations across all these agencies, creating compounding legal exposure that extends far beyond the individual employee.
The High-Stakes Nature of Pharmaceutical Employment
Pharmaceutical sales representatives occupy a particularly sensitive position within this regulated environment. These professionals routinely access medication samples worth thousands of dollars. They enter healthcare facilities without direct supervision. They influence prescribing decisions that directly impact patient outcomes. The combination of sample access, limited oversight, and financial incentives creates fraud and diversion risks absent in most other industries.
Recent enforcement data reveals the scope of this risk. Between 2020 and 2024, the OIG excluded over 7,200 individuals from federal healthcare programs. Pharmaceutical industry violations represented the fastest-growing category. The average financial penalty for companies employing excluded individuals now exceeds $2.8 million per case, not including the reputational damage and ongoing monitoring requirements that follow.
Beyond Criminal Background Checks: Understanding Pharma-Specific Risks
Standard employment background checks typically verify criminal history, employment dates, and educational degrees. While these elements matter in pharmaceutical hiring, they represent only a fraction of the compliance picture. Pharmaceutical background checks must address several specialized risk areas that conventional screening overlooks.
Consider the gaps that standard screening creates. A candidate might pass a conventional background check while simultaneously appearing on state medical board disciplinary lists. They could be listed in federal exclusion databases. They might appear in healthcare fraud monitoring systems. This disconnect between standard screening and pharmaceutical employee screening requirements has led to numerous high-profile compliance failures and resulting penalties.
The Essential Pharma Sales Rep Screening Checklist
Creating an effective pharma sales rep screening checklist requires understanding both baseline compliance requirements and industry best practices. The pharmaceutical industry faces unique verification challenges that demand comprehensive evaluation across multiple risk dimensions. Standard background checks address general employment suitability but miss critical pharmaceutical-specific concerns.
The following components represent the essential elements that pharmaceutical companies must implement. Each component addresses specific regulatory requirements or industry risk factors. Together, they create the comprehensive approach necessary for adequate pharmaceutical industry hiring compliance.
Criminal History and Fraud Investigation Components
Pharmaceutical companies must conduct thorough criminal background searches covering specific offense categories. The screening process should examine all jurisdictions where candidates have lived or worked during the past seven years. This timeframe aligns with FCRA guidelines while providing sufficient lookback to identify relevant patterns.
| Screening Component | Scope and Requirements | Risk Addressed |
| Seven-Year Criminal Search | Federal and state databases across all residence/work jurisdictions | Healthcare fraud, controlled substance violations, theft offenses |
| Financial Crime Review | Bankruptcy filings, tax liens, civil judgments, fraud convictions | Sample diversion risk, financial pressure indicators |
| Motor Vehicle Records | DUI/DWI violations, suspended licenses, reckless driving | Substance abuse indicators, judgment concerns |
Pay particular attention to fraud-related offenses, theft, controlled substance violations, healthcare crimes, and financial crimes. Even misdemeanor convictions in these categories warrant careful evaluation. They may indicate risk patterns relevant to pharmaceutical employment regardless of the offense level.
Healthcare-Specific Sanctions and Exclusions
Federal exclusion list screening represents the single most critical component of pharmaceutical employee screening requirements. Companies participating in federal healthcare programs cannot employ excluded individuals in any capacity. Violations result in severe penalties including program exclusion, substantial fines, and potential criminal prosecution.
The verification process must address multiple specialized databases:
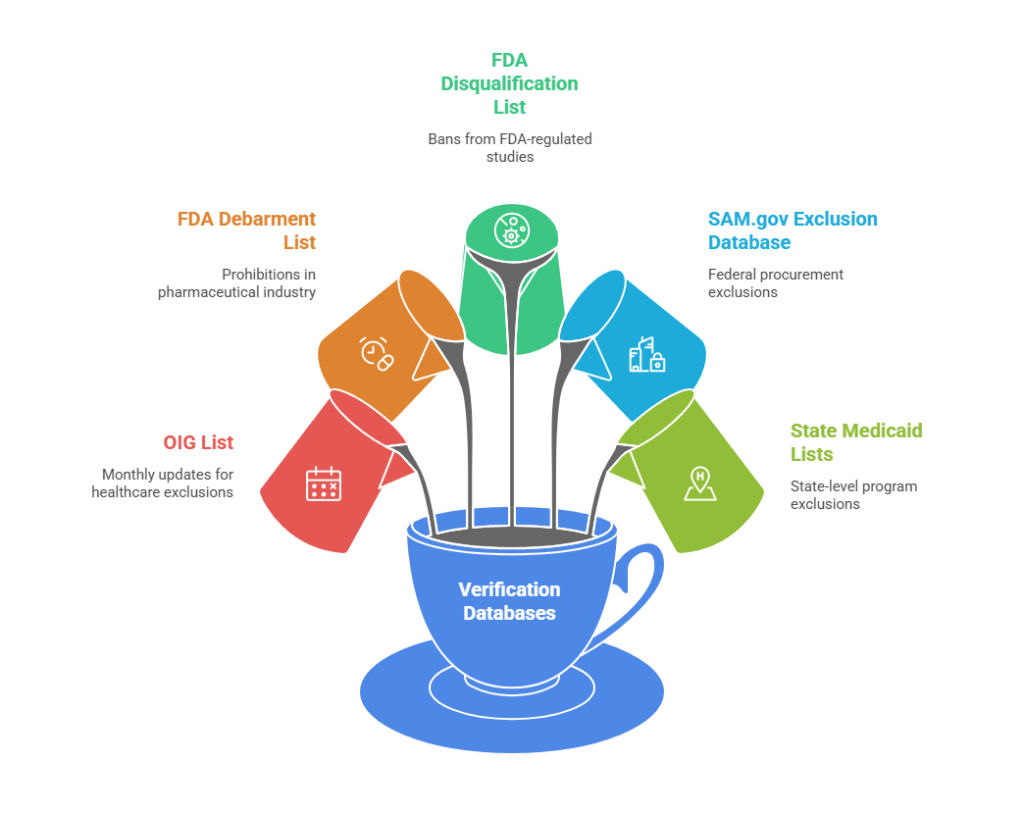
- OIG List of Excluded Individuals and Entities (LEIE): Updated monthly with exclusions for patient abuse, healthcare fraud, controlled substance violations, and license revocations
- FDA Debarment List: Individuals prohibited from pharmaceutical manufacturing, drug application processes, or industry employment
- FDA Clinical Investigator Disqualification List: Researchers banned from conducting FDA-regulated studies
- SAM.gov Exclusion Database: Federal procurement and non-procurement exclusions affecting government contracting
- State Medicaid Exclusion Lists: State-level program exclusions that may precede federal action
OIG screening cannot be a one-time event. New exclusions appear monthly, and approximately 34% of all exclusions occur after initial employment. Pharmaceutical industry hiring compliance demands monthly rescreening of all employees, contractors, and vendors with any connection to federal healthcare programs.
FDA Compliance Screening: Navigating Regulatory Requirements

FDA compliance screening encompasses verification processes specifically designed to ensure pharmaceutical employees meet federal regulatory standards. These requirements reflect the FDA's unique oversight role in pharmaceutical manufacturing, distribution, and marketing. Unlike general employment screening, FDA compliance focuses on regulatory history and professional credentials that directly impact company operations.
The pharmaceutical sector operates under FDA scrutiny that extends beyond products to the people who represent them. Sales representatives serve as the direct link between pharmaceutical companies and healthcare providers. Their credentials, history, and ongoing compliance status affect corporate regulatory standing in ways that standard employment relationships do not.
Controlled Substance and DEA Registration Verification
Pharmaceutical companies distributing controlled substances operate under DEA registration requirements. While not all pharmaceutical sales representatives require individual DEA registrations, companies must verify specific compliance factors. Employees with sample access must meet DEA security requirements. They cannot have histories of controlled substance violations that could jeopardize company registrations.
DEA database searches should identify several critical issues:
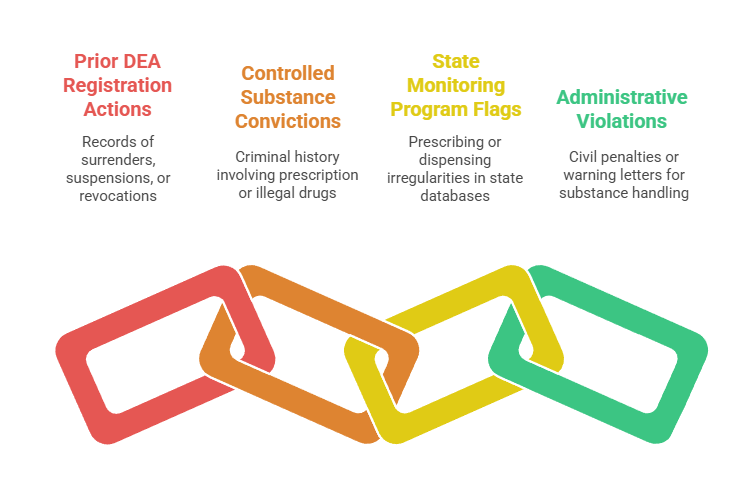
- Prior DEA Registration Actions: Surrenders, suspensions, or revocations indicating serious compliance failures
- Controlled Substance Convictions: Criminal history involving prescription drugs, illegal drugs, or diversion
- State Monitoring Program Flags: Prescribing or dispensing irregularities in state-level databases
- Administrative Violations: Civil penalties or warning letters related to controlled substance handling
These searches prove particularly valuable because they identify candidates unsuitable for pharmaceutical employment regardless of the specific role. A history of DEA violations indicates integrity concerns that transcend job descriptions. State-level controlled substance monitoring programs often contain information not yet reflected in federal databases, providing early warning of emerging compliance concerns.
Professional License and Credential Verification
Healthcare license primary source verification stands as a non-negotiable requirement for pharmaceutical hiring compliance. Companies must verify licenses directly with issuing boards rather than relying on candidate-provided documentation. This verification applies to pharmacy licenses, nursing licenses, medical licenses, and all clinical credentials.
| License Type | Verification Source | Key Items to Confirm |
| Medical Licenses | State Medical Boards | Active status, disciplinary actions, practice restrictions, malpractice history |
| Pharmacy Licenses | State Boards of Pharmacy | Current registration, controlled substance authority, complaint history |
| Nursing Licenses | State Nursing Boards | Multi-state license status, scope limitations, rehabilitation agreements |
| DEA Registrations | DEA Diversion Control | Active status, schedule authority, facility restrictions |
License disciplinary actions often indicate integrity or competency concerns relevant to pharmaceutical employment. Many state licensing boards maintain publicly accessible databases, but some require direct contact for complete disclosure. Verification should confirm current active status, absence of disciplinary actions, restrictions on practice, and history of complaints or investigations.
Healthcare Worker Verification: Multi-Jurisdictional Complexity
Healthcare worker verification for pharmaceutical industry purposes requires coordinating searches across fragmented database systems. The United States operates without centralized healthcare professional licensing or disciplinary tracking. This fragmentation creates gaps that inadequate screening processes fail to address. Pharmaceutical companies face vulnerability when verification stops at surface-level checks.
The complexity stems from jurisdictional separation between federal and state oversight systems. Each maintains distinct databases with non-standardized formats and varying disclosure requirements. Comprehensive verification demands systematic searching across all relevant authorities. Missing even one jurisdiction can leave critical compliance gaps that expose companies to regulatory enforcement.
Navigating State Medical Board Databases
State medical board searches must cover all jurisdictions where candidates have worked, lived, or held licenses. The United States operates 70+ separate medical boards with non-standardized database systems. Some boards provide comprehensive online disclosure. Others require written requests or direct contact for complete disciplinary history information.
Medical board searches identify several critical concern areas:
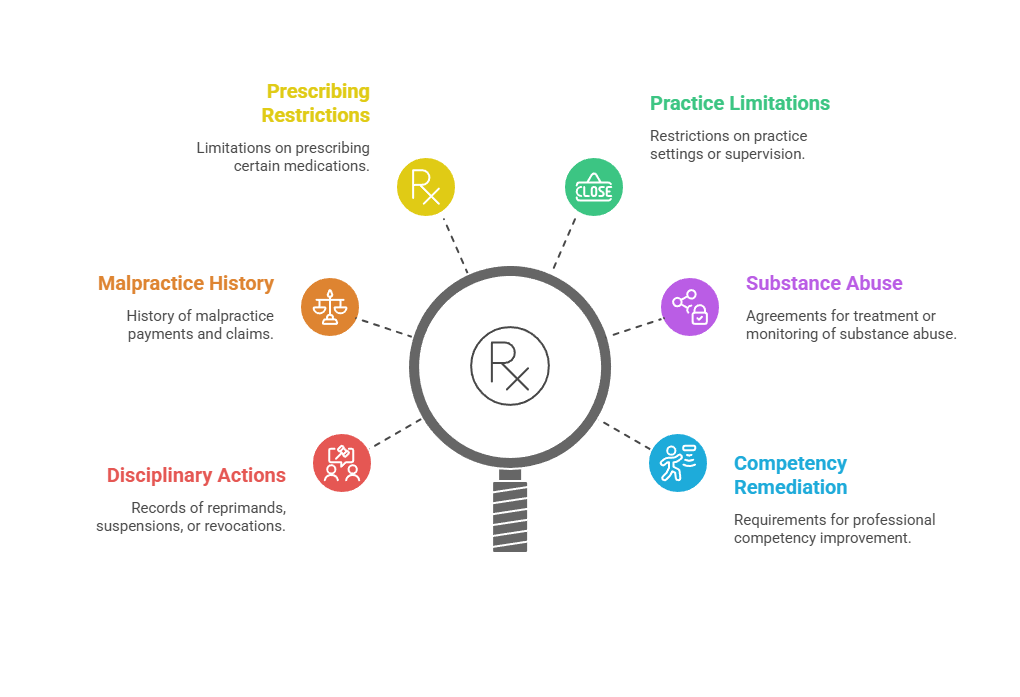
- License disciplinary actions including reprimands, suspensions, and revocations
- Malpractice payment history through the National Practitioner Data Bank where accessible
- Restrictions on prescribing authority or specific medication classes
- Practice setting limitations or supervisory requirements
- Substance abuse treatment or monitoring agreements
- Professional competency remediation requirements
These disciplinary histories frequently relate to integrity concerns directly relevant to pharmaceutical employment suitability. A prescribing restriction might indicate controlled substance diversion concerns. Substance abuse monitoring agreements suggest risks for positions involving medication sample access. Professional competency issues could affect a medical science liaison's ability to provide accurate clinical information.
Professional Association and Certification Verification
Many pharmaceutical sales representatives hold professional certifications from industry associations. While these certifications aren't legally required, they often appear prominently on resumes and influence hiring decisions. Verification ensures candidates actually hold claimed certifications. It confirms that issuing organizations maintain proper accreditation and ethical standards.
The verification process should examine certification authenticity and current status. Professional associations sometimes revoke certifications for ethics violations or continued education failures. These revocations may indicate integrity concerns not visible in criminal background checks or license verification. Primary source confirmation directly with certifying organizations prevents resume fraud and identifies ethical issues that affect employment suitability.
Building Ongoing Monitoring into Pharmaceutical Employee Screening Requirements
The most sophisticated pharmaceutical industry hiring compliance programs recognize that screening cannot end at the hiring decision. Ongoing monitoring addresses a critical reality: approximately one-third of all exclusions, sanctions, and license disciplinary actions occur after initial employment. This post-hire risk creates compliance vulnerabilities that initial screening cannot prevent, regardless of thoroughness.
Federal enforcement statistics demonstrate the necessity of continuous monitoring. New OIG exclusions appear monthly, averaging 150-200 additions. FDA debarment actions occur throughout the year without predictable patterns. State licensing boards take disciplinary actions continuously, often months or years after investigations begin. Without ongoing monitoring, pharmaceutical companies operate with outdated compliance information that grows increasingly unreliable over time.
Monthly Database Rescreening Protocols
Best practice pharmaceutical employee screening requirements include monthly automated rescreening against multiple databases. This frequency aligns with OIG database update schedules and represents the industry standard for adequate ongoing monitoring. Monthly rescreening should cover all employees, not merely those in patient-facing or sample-access roles.
The comprehensive monthly rescreening process should include:
- OIG Exclusion List: Primary federal healthcare program exclusion database updated monthly
- FDA Debarment List: Pharmaceutical industry-specific prohibitions on employment or participation
- SAM.gov Exclusions: Federal procurement and assistance program restrictions
- State Medicaid Exclusions: Individual state program exclusions that may precede federal action
- GSA Suspension/Debarment: General Services Administration actions affecting federal contracting
Federal exclusion triggers program participation consequences regardless of the excluded individual's specific job responsibilities. Administrative staff, executives, sales representatives, and clinical personnel all create identical liability when excluded. This company-wide risk makes universal monthly monitoring essential rather than position-specific screening.
Event-Triggered Immediate Rescreening
Beyond routine periodic monitoring, certain triggering events demand immediate comprehensive rescreening. These events indicate elevated risk requiring urgent verification even for recently screened employees. Pharmaceutical companies should establish clear internal protocols defining triggering events and mandating immediate action when they occur.
| Triggering Event | Rescreening Scope | Typical Timeline |
| Arrest or Criminal Charges | Full criminal background, relevant licensing boards | Within 48 hours of notification |
| Civil Healthcare Lawsuit | OIG/FDA/SAM databases, professional licenses | Within 5 business days |
| License Suspension Notice | All professional licenses held, related board actions | Immediate upon notification |
| Performance/Integrity Concerns | Complete baseline screening refresh | Within 10 business days |
This event-driven approach supplements periodic monitoring by addressing acute risks as they emerge. Waiting for the next scheduled verification cycle when red flags appear creates unnecessary compliance exposure. Immediate rescreening upon triggering events enables swift corrective action before regulatory violations crystallize or worsen.
The True Cost of Inadequate Pharmaceutical Background Checks

Understanding the financial and operational consequences of inadequate pharmaceutical background checks helps justify comprehensive screening investments. These costs extend far beyond immediate penalties for compliance violations. They encompass long-term operational restrictions, reputational damage, and business opportunity losses that persist years after resolution.
Federal enforcement statistics reveal the severity of pharmaceutical industry hiring compliance failures. Between 2020 and 2024, average settlement amounts for cases involving employment of excluded individuals exceeded $2.8 million. These figures represent only direct monetary penalties. Legal fees, investigation costs, and remediation expenses typically add 40-60% to total enforcement costs, creating actual financial impacts exceeding $4 million per case.
Regulatory Penalties and Corporate Integrity Agreements
Direct financial penalties represent just the beginning of enforcement consequences. Beyond monetary settlements, enforcement actions frequently result in Corporate Integrity Agreements imposing five-year monitoring requirements. These CIAs mandate compliance program enhancements, independent review obligations, and reporting requirements costing millions annually throughout their duration.
Corporate Integrity Agreements essentially place companies under federal probation with extensive obligations:
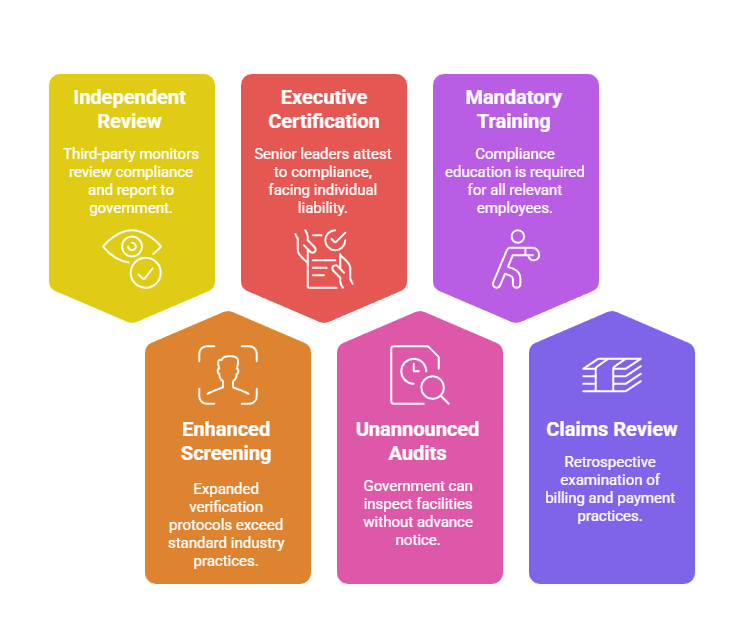
- Independent Review Organizations: Third-party monitors reviewing compliance systems and reporting directly to government agencies
- Enhanced Screening Requirements: Expanded verification protocols exceeding standard industry practices
- Executive Certification: Personal attestation by senior leaders regarding compliance status with potential individual liability
- Unannounced Audits: Government rights to inspect facilities, records, and operations without advance notice
- Mandatory Training: Specified compliance education for all relevant employees with documentation requirements
- Claims Review: Retrospective examination of billing and payment practices with repayment obligations
The operational burden of CIAs disrupts normal business operations throughout their five-year term. Companies under CIAs face increased regulatory scrutiny for all activities, not merely hiring compliance. This heightened oversight creates cascading effects on business efficiency, strategic flexibility, and competitive positioning.
Reputational Damage and Market Access Consequences
Pharmaceutical companies operate in a reputation-sensitive industry where trust represents a core business asset. Public disclosure of hiring compliance failures damages relationships with healthcare providers, patients, investors, and business partners. These reputation effects persist long after regulatory penalties are paid and formal enforcement actions conclude.
Healthcare systems increasingly conduct vendor due diligence before granting pharmaceutical representatives facility access. Companies with recent compliance violations often face access restrictions or complete exclusion from prestigious healthcare institutions. Sales representatives may require additional oversight, face limitations on unsupervised provider interactions, or lose access privileges entirely. These restrictions directly impact sales effectiveness and market position in ways that financial penalties alone cannot measure.
Implementing Your Pharmaceutical Industry Hiring Compliance Program
Translating pharmaceutical employee screening requirements from conceptual understanding to operational reality requires systematic implementation. Success depends on treating compliance as an integrated business process rather than an isolated HR function. Technology systems, staff training, vendor partnerships, and ongoing program management must work cohesively to deliver reliable compliance outcomes.
Most pharmaceutical companies partner with specialized background screening vendors rather than conducting all verification internally. Generic background screening vendors often lack access to specialized healthcare databases. They may not understand pharmaceutical industry risk factors. Their staff typically lacks experience interpreting complex exclusion and licensing information that pharmaceutical compliance demands.
Vendor Selection and Technology Integration
Vendor selection should prioritize demonstrated pharmaceutical industry experience and healthcare database access. The cost differential between specialized pharmaceutical screening vendors and generic providers proves minimal compared to risk reduction benefits. Technology platforms should support workflow automation, audit trail documentation, policy enforcement, and compliance reporting.
Modern applicant tracking systems integrate with background screening platforms to create seamless workflows. These integrations ensure no candidate progresses to employment without completing all required verification steps. Automated workflows eliminate human error in compliance processes while creating comprehensive documentation of screening completion. Integration also enables efficient ongoing monitoring by automatically triggering periodic rescreening on predetermined schedules.
Adjudication Standards and Training Requirements
Comprehensive screening inevitably identifies concerning information requiring human judgment about hiring suitability. Clear, documented adjudication standards must define how various findings are evaluated and who makes final employment decisions. These standards should address common scenarios: criminal convictions of various types and ages, financial history concerns, license disciplinary actions, and conflicting information between sources.
Training for hiring managers and recruiters must emphasize why pharmaceutical screening differs from general employment verification. Effective training addresses what specific risks the company faces from inadequate screening. It explains how proper processes protect both the company and individual managers from liability. Annual refresher training ensures compliance awareness remains current as regulations evolve and new staff join hiring roles.
Conclusion
Pharmaceutical industry hiring compliance represents a complex, high-stakes process where standard background screening proves dangerously inadequate. The unique combination of controlled substance access, healthcare provider interactions, and stringent regulatory oversight demands specialized screening addressing OIG exclusions, FDA compliance, healthcare sanctions, and ongoing monitoring. With average penalties exceeding $2.8 million per violation and enforcement actions increasing annually, pharmaceutical companies must implement comprehensive screening programs protecting patient safety and corporate viability. The investment in proper pharmaceutical employee screening requirements delivers enormous returns through regulatory compliance, risk mitigation, and operational certainty that inadequate screening can never achieve.
Frequently Asked Questions
What makes pharmaceutical background checks different from standard employment screening?
Pharmaceutical background checks extend beyond criminal history to include mandatory OIG exclusion list verification, FDA debarment searches, and healthcare license verification. These specialized searches address unique pharmaceutical industry risks related to sample access and healthcare provider interactions. Multi-database searching covers federal and state sanctions that standard employment screening typically misses.
How often must pharmaceutical companies screen employees against the OIG exclusion list?
Pharmaceutical companies must screen all employees against the OIG exclusion list before hiring and at least monthly thereafter. The OIG updates its exclusion database monthly, and approximately 34% of all exclusions occur after initial employment. Monthly monitoring is essential for compliance rather than optional best practice.
What are the penalties for employing an individual on the OIG exclusion list?
Companies employing excluded individuals face potential penalties of $10,000 per excluded person per claim submitted to federal healthcare programs. Additional consequences include exclusion from Medicare and Medicaid participation, refund obligations for payments received while the excluded individual was employed, and potential False Claims Act liability with treble damages. Recent enforcement actions average $2.8 million in total penalties per case.
Do pharmaceutical sales representatives need individual DEA registrations?
Most pharmaceutical sales representatives do not require individual DEA registrations, as they typically operate under their employer's corporate registration. However, companies must verify that sales representatives with controlled substance sample access have no history of DEA violations or controlled substance convictions. These factors could jeopardize corporate DEA registration status or create diversion risks.
What specific databases should pharmaceutical companies search during employee screening?
Comprehensive pharmaceutical employee screening should search the OIG LEIE database, FDA Debarment List, FDA Clinical Investigator Disqualification List, and SAM.gov exclusion database. Additional searches should include state Medicaid exclusion lists, state medical and pharmacy board databases, DEA registration databases, and relevant professional licensing boards. Multi-database searching is essential because no single database captures all relevant exclusions and sanctions.
How long should pharmaceutical companies retain background check documentation?
Pharmaceutical companies should retain background check documentation for the duration of employment plus at least seven years after separation. This timeframe aligns with False Claims Act statute of limitations periods. In cases involving OIG exclusion list screening, permanent retention is advisable as companies may need to demonstrate historical compliance during investigations occurring years later.
Can pharmaceutical companies hire individuals with criminal records?
Pharmaceutical companies may hire individuals with certain criminal records depending on the nature, age, and relevance of convictions. However, companies should exercise extreme caution with healthcare fraud, controlled substance violations, theft, financial crimes, and patient abuse convictions. Individualized assessment considering job responsibilities and rehabilitation evidence is required under EEOC guidance, but pharmaceutical industry risks often justify exclusion of candidates with relevant criminal histories.
What ongoing monitoring is required after initial pharmaceutical employee screening?
Ongoing monitoring should include monthly OIG and FDA database rescreening, quarterly professional license status verification, and annual comprehensive background updates. Immediate rescreening is required upon triggering events such as arrests, civil litigation, or performance issues suggesting integrity concerns. Approximately one-third of all sanctions and exclusions occur post-hire, making ongoing monitoring as critical as initial screening.
Additional Resources
- OIG List of Excluded Individuals and Entities (LEIE) Database
https://oig.hhs.gov/exclusions/ - FDA Debarment List - Individuals and Entities
https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/compliance-actions-and-activities/drug-product-application-approval-and-related-actions-debarment-list - HHS Compliance Program Guidance for Pharmaceutical Manufacturers
https://oig.hhs.gov/compliance/compliance-guidance/ - DEA Practitioner and Distributor Registration Resources
https://www.deadiversion.usdoj.gov/drugreg/ - Federation of State Medical Boards - Physician License Verification
https://www.fsmb.org/fcvs/ - SAM.gov Federal Exclusion Database
https://www.sam.gov/SAM/
